
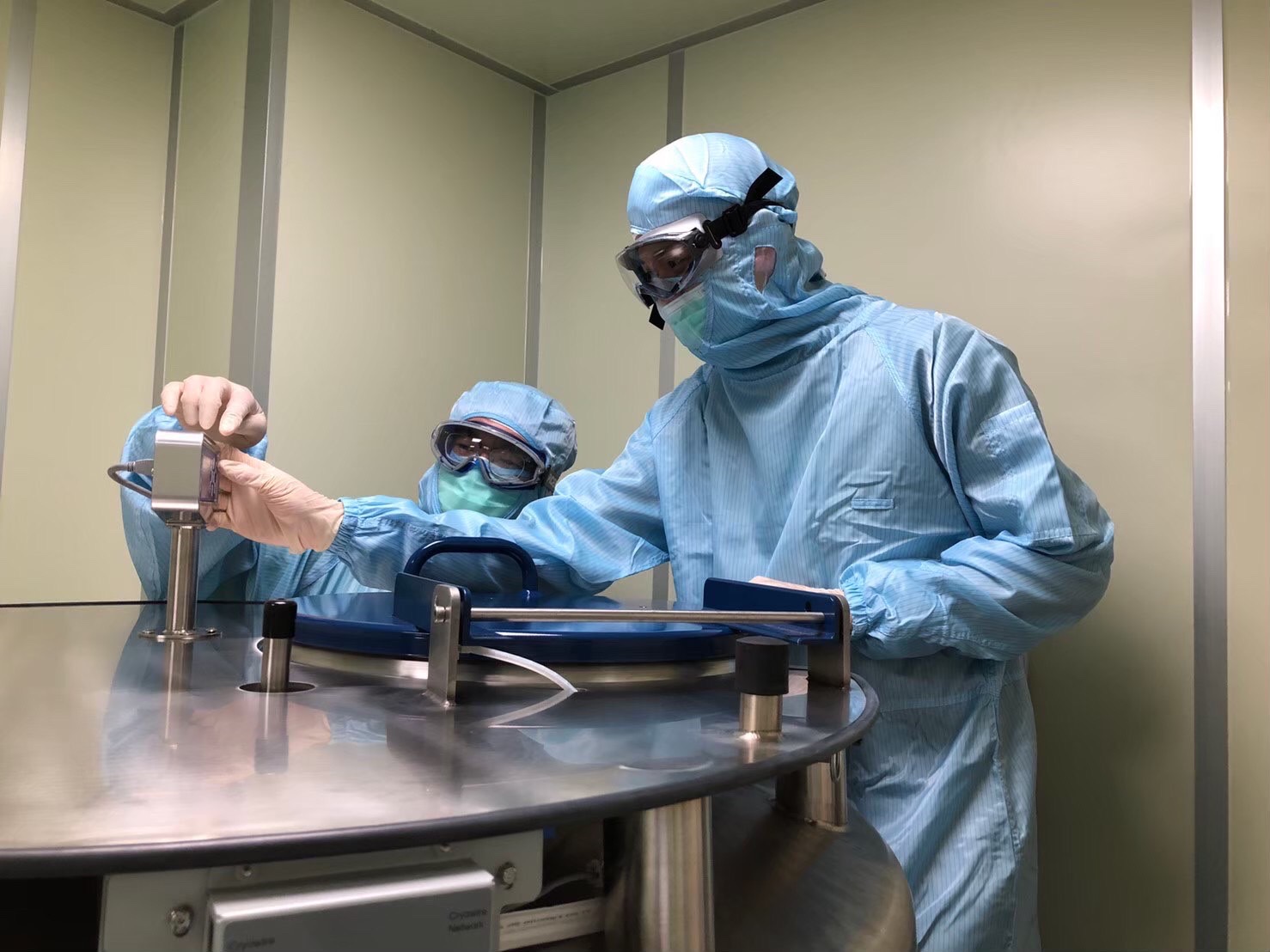
The Cell Therapy Center at E-Da Hospital is certificated as a cell processing unit for cell manufacturing, storage and clinical trials (Photo by E-Da Hospital)
The Cell Therapy Center and integrated cancer care are two distinctive features of E-Da Healthcare Group (hereinafter E-Da Hospital). Anticipating multiple certifications from the Taiwan Food and Drug Administration by the end of 2023, E-Da is set to showcase its Cancer Center services, peripheral blood stem cell transplantation, cancer gene NGS testing, and GTP laboratory business at the 2023 Healthcare+ Expo. In future collaborations with the industry, E-Da aims to provide end-to-end services, including manufacturing, quality control, storage, and clinical trials, ensuring comprehensive validation for emerging technologies.
GTP Cell Laboratory: One-stop Process from Manufacturing, Quality Control, Storage to Clinical Trials
Since the establishment of the Cell Therapy Center at E-Da Hospital, initiated by President Yuan-KunTu in 2018, the hospital has pioneered the world's first project using chondrocyte sheets to treat knee cartilage defects. As of September 2023, within three years, the center has successfully enrolled 63 patients, setting a global record for the highest enrollment and success rates of treatment. The project is still ongoing.
E-Da’s GTP Cell Laboratory started trial operations in 2020 and achieved ISO 17025 certification for quality control inspection in December of the same year. In 2023, the laboratory underwent inspections and accreditation from the Taiwan Food and Drug Administration for the Cell Processing Unit (CPU) and the peripheral blood stem cell (PBSC) storage, with certification expected to be obtained by the end of 2023. Once certified, it will mark the completion of the first comprehensive certification process from manufacturing and quality control to storage, a rare achievement among medical centers in Taiwan.
In the future, E-Da Hospital can assist academic and research institutions as well as industries in translating or transferring technology. Whether it's immune cells, mesenchymal stem cells, cell derivatives, or exosomes, E-Da has the capability to integrate laboratory research results into the process of cell product development. Coupled with the hospital's clinical service capacity and a well-established clinical trial system, E-Da can further advance the clinical validation of cell products.
E-da has been approved for Taiwan’s first hospital to repair knee cartilage defects with chondrocyte sheet transplantation as early as in 2018 (Photo by E=Da Hospital)
The Hospital Formed 11 Cancer Care Teams and Approved for Clinical Trials in Southern Taiwan
In the field of integrated cancer care, E-Da Hospital has established 11 cancer care teams, including specialists in surgical oncology, medical oncology, radiation oncology, diagnostic imaging, pathology, and other fields. The teams also include case managers and nutritionists. E-Da has introduced state-of-the-art equipment such as the fourth-generation da Vinci surgical system, a 3T high-field magnetic resonance imaging (MRI) machine, and an advanced 640-slice computed tomography (CT) scanner. This integrated approach covers over 95% of cancer patients throughout the entire hospital.
The team utilises cancer tests for assessing and predicting cancer treatment outcomes. This approach allows patients to benefit from more accurate diagnosis, medication, and monitoring. Additionally, the hospital integrates complementary therapies such as traditional Chinese medicine, integrative medicine, palliative care, and the cell therapy center. This comprehensive approach, combined with tailored dietary adjustments and rehabilitation, provides patient-centric services within an intelligent healthcare environment.
In 2022, E-Da Cancer Hospital established a Hematopoietic Stem Cell Transplantation Center, aiming to obtain yet another accreditation from the Food and Drug Administration for a PBSC bank by the end of 2023. The center has successfully completed several cases of autologous hematopoietic stem cell transplantation and is moving towards allogeneic hematopoietic stem cell transplantation. Additionally, E-Da Cancer Hospital actively participates in clinical trials for new drugs, making it one of the few hospitals in the Kaohsiung area involved in new drug testing.
Immune Cell Function Screening Tests Supporting CIK Cell Therapy
In order to select cancer patients suitable for CIK cell therapy and avoid blind recruitment leading to ineffective treatment, E-Da’s Cell Therapy Center has developed its immune cell function screening platform based on the test data of hundreds of patients. This platform represents the convergence of two distinctive features E-Da has to offer.
The platform assesses the immune system of cancer patients in advance to ensure whether the patient physically qualifies for CIK cell therapy, achieving the goal of personalised medicine. Beyond cancer patients, the platform can also be applied to health checkups, evaluating the immune system of healthy individuals and providing comprehensive health information and maintenance recommendations. Currently, E-Da Hospital hopes to expand clinical trials to expedite the commercialisation of the technology it has developed.

E-Da’s immune cell function screening platform is actively taking part in clinical trials, in hopes that technology involved can be commercialised (Photo by E-Da Hospital)
★★★More by E-Da Healthcare Group
◎Advanced Endoscopic Diagnosis and Treatment Center
Of the top ten causes of cancer deaths in Taiwan, five are related to gastrointestinal cancers, including colorectal, pancreatic, gastric and oesophageal cancers, all of which require endoscopy. In recognition of this, E-Da Cancer Hospital will establish the International Advanced Endoscopic Diagnosis and Treatment Center, which is expected to operate in April 2024. The center will offer ten advanced endoscopic treatments, including radiofrequency ablation (RFA) for oesophageal precancerous lesions. With resources and referrals, the center aims to provide the highest quality service for international medical service. The hospital welcomes collaboration with various companies to use it as a testing ground for niche medical devices.
◎Advanced Gastroesophageal Reflux Center
Every year, around 760,000 people in Taiwan seek medical care for gastroesophageal reflux disease (GERD). In response to increasing GERD cases, the Advanced GERD Diagnosis and Treatment Center will officially open at E-Da Hospital in January 2024. The center will integrate a comprehensive GERD diagnosis and treatment team, develop new treatment technologies for GERD, and collaborate with the Wellness Center for dietary education and the promotion of precision medicine.
Additionally, the center will assist other hospitals in handling challenging cases and use radiofrequency ablation to treat precancerous lesions caused by GERD (Barrett's esophagus). It aims to further develop international medical services and become a designated referral center for GERD in Southeast Asia.
B2B Opportunities with E-Da Healthcare Group
Precision Medicine
| Products/Solutions | Partnership wanted |
|---|---|
| Advanced gastroesophageal reflux center | International medical service agents |
| NGS testing for cancer genes | Technology transfer; product development |
Regenerative Medicine
| Products/Solutions | Partnership wanted |
|---|---|
| GTP Cell Laboratory | Technology transfer; product development |
| Peripheral Blood-derived Stem Cell Transplantation | Technology transfer; product development |
Innovative R&D
| Products/Solutions | Partnership wanted |
|---|---|
| CIK Cell Therapy approved project and immune cell function screening platform | Research collaboration |
New and About
| Products/Solutions | Partnership wanted |
|---|---|
| Advanced endoscopic diagnosis and treatment center provides therapy such as Radiofrequency Ablation (RFA) for precancerous lesions in esophageal cancer | International medical service agents |
E-Da Healthcare Group at Healthcare+ Expo 2023
- Thursday, 30 November to Sunday, 3 December
- Booth No. J502 | 1F, TaiNEX 1
