
New cell drug company on all therapies-Ever Supreme Biotechnology Co., LTD.
2020-10-28
Cell Therapy CMO:
Ever Supreme offers the cell products (ADCV、DC-CIK、CIK、GDT and BMSC) needed by medical institution under the Regulations Governing Specific Cellular Therapeutic Technology of 2018 promogulated by Taiwan FDA.
Cells Products as CMO:
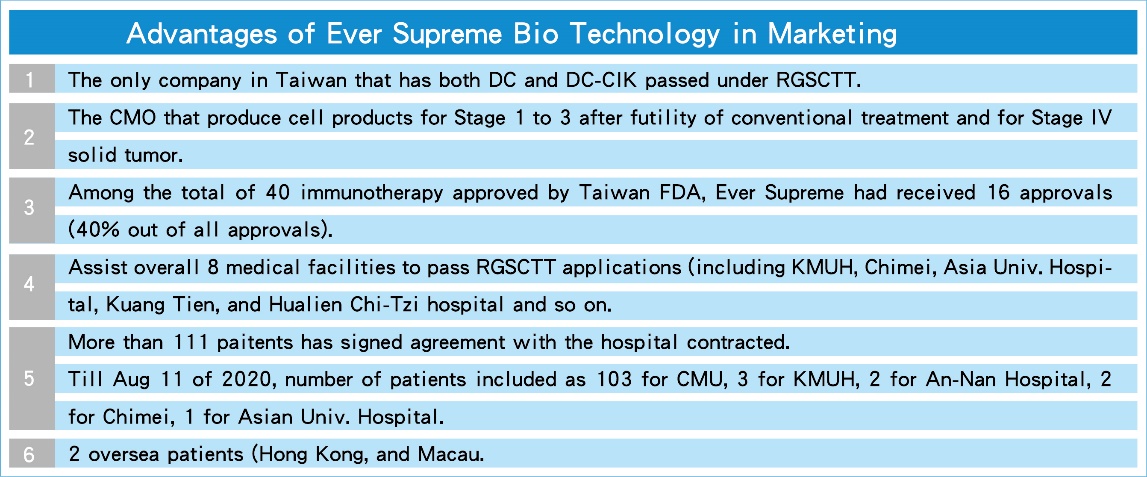
(1).Advantages of Ever Supreme Bio Technology in Marketing:
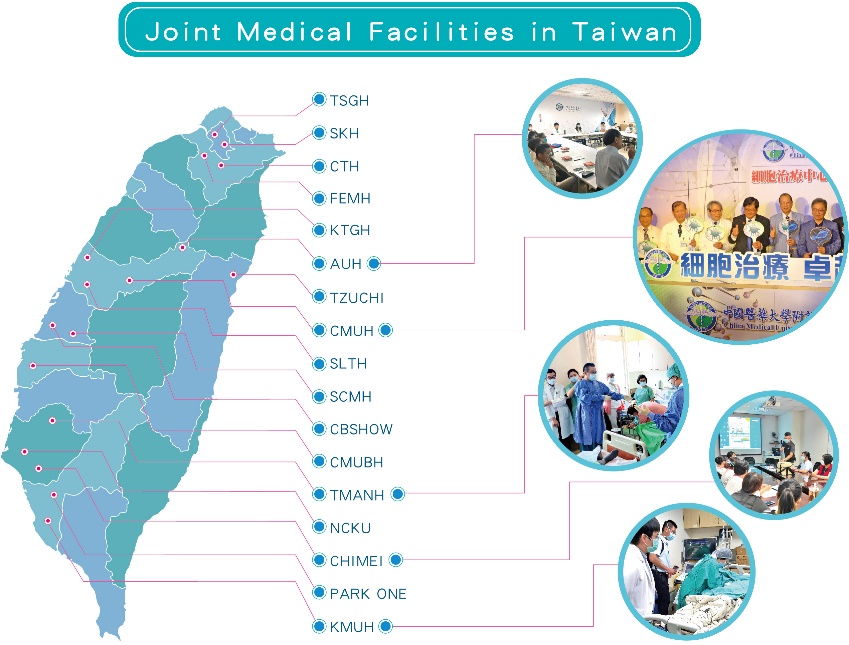
(2).Joint Medical Facilities in Taiwan
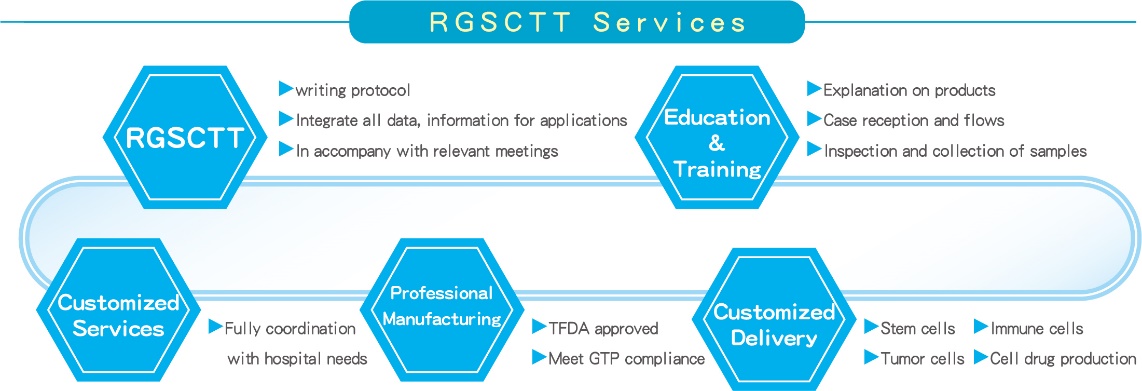
(3).RGSCTT Services
(4). Indications of Treatment by Ever Supreme under RGSCTT
(5). Clinical Data under RGSCTT (ADCV01)
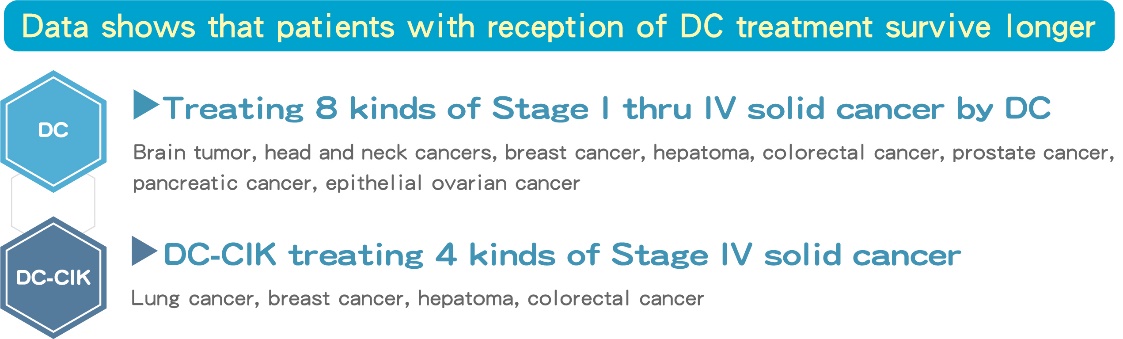
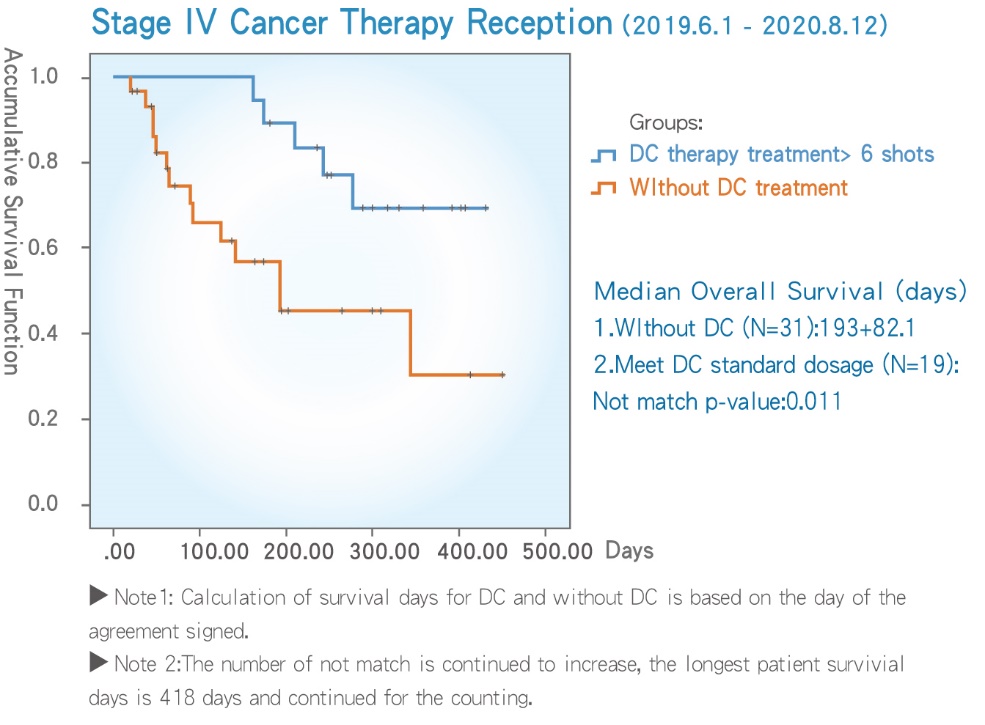
■ Data shows that patients with reception of DC treatment survive longer.
- 19 cases on reception of DC therapy with 6 or more vails show survival days for at least 260 days and above, the longest survival days had exceeded more than 418 days (and continues to be rise).
- 31 cases on reception of DC therapy with greater than 6 vails have significantly shown a higher impact than without treatment, showed a survival day of 193±82.1 days, the p-value in between is 0.011, a data demonstrates longer survival time.
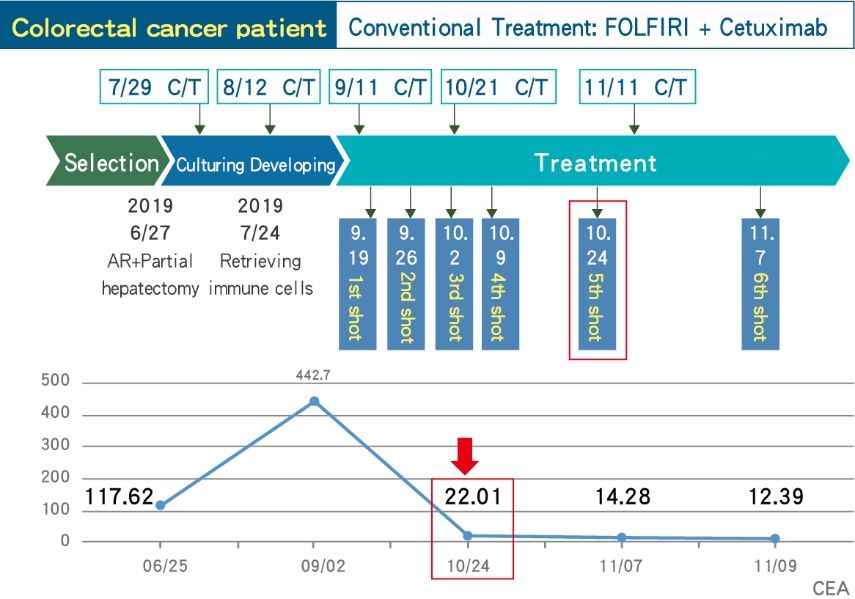
■ A Stage IV colorectal cancer patient who had received DC therapy, whose tumor marker are drastically decreased and maintain stable.
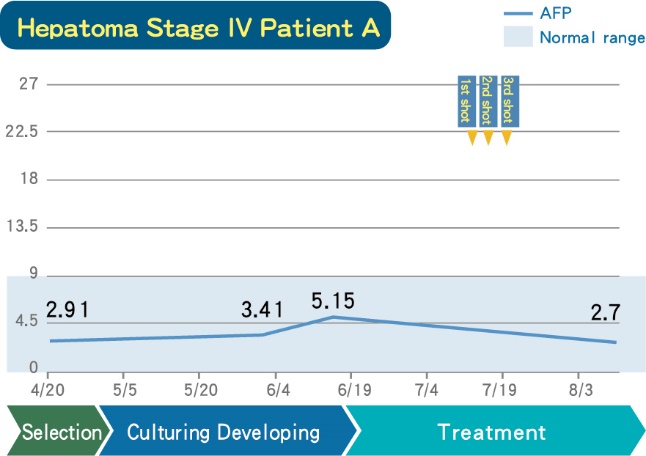
(6). Clinical Data under RGSCTT(DC-CIK)
■ A Stage IV Hepatoma patient who had received DC-CIK therapy, whose tumor marker are maintained stable range, no deterioration.
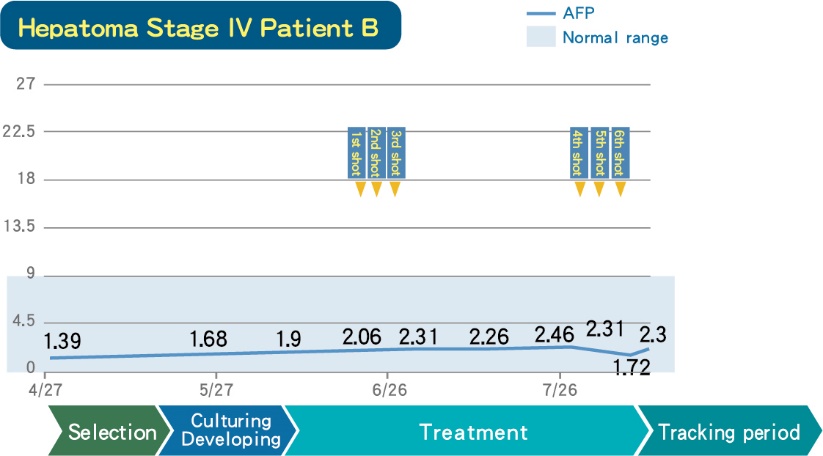
■ A Stage IV Hepatoma patient who had received DC-CIK therapy, whose tumor marker are maintained stable range, no deterioration.
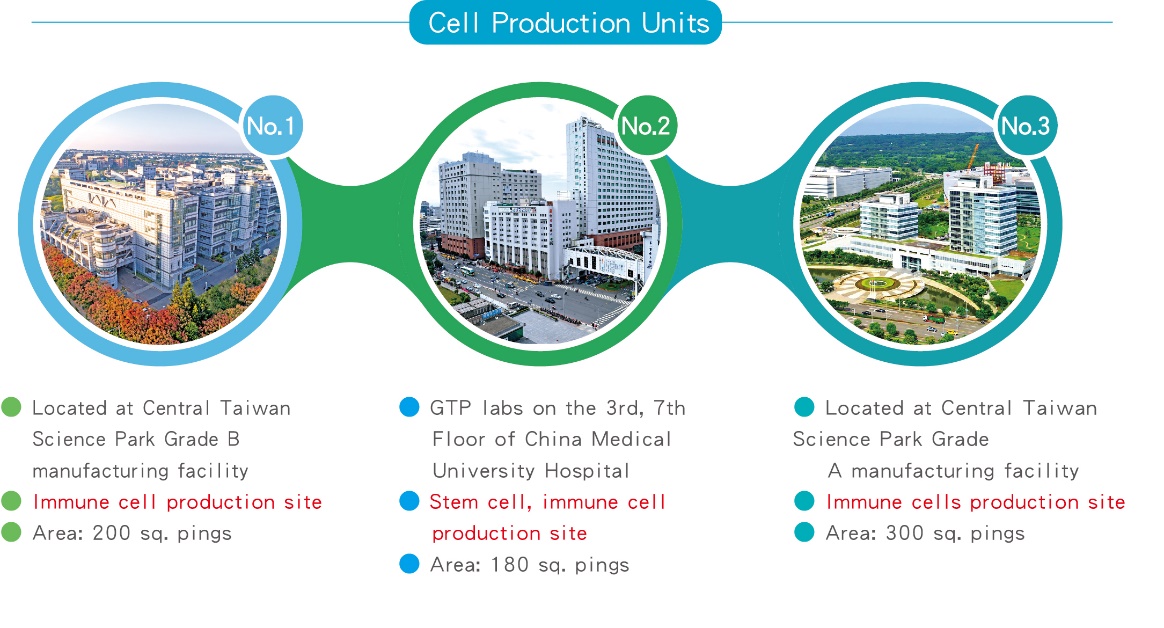
4.CPU sites
Look for
Product licensing, Joint-Venture, Strategic Alliance, Sales & Distribution
Booth No. M804