FP-025:Potential Treatment of COVID-19-Induced Acute Respiratory Distress Syndrome
2020-11-09
FP-025:Potential Treatment of COVID-19-Induced Acute Respiratory Distress Syndrome
Foresee Pharmaceuticals Co., Ltd.
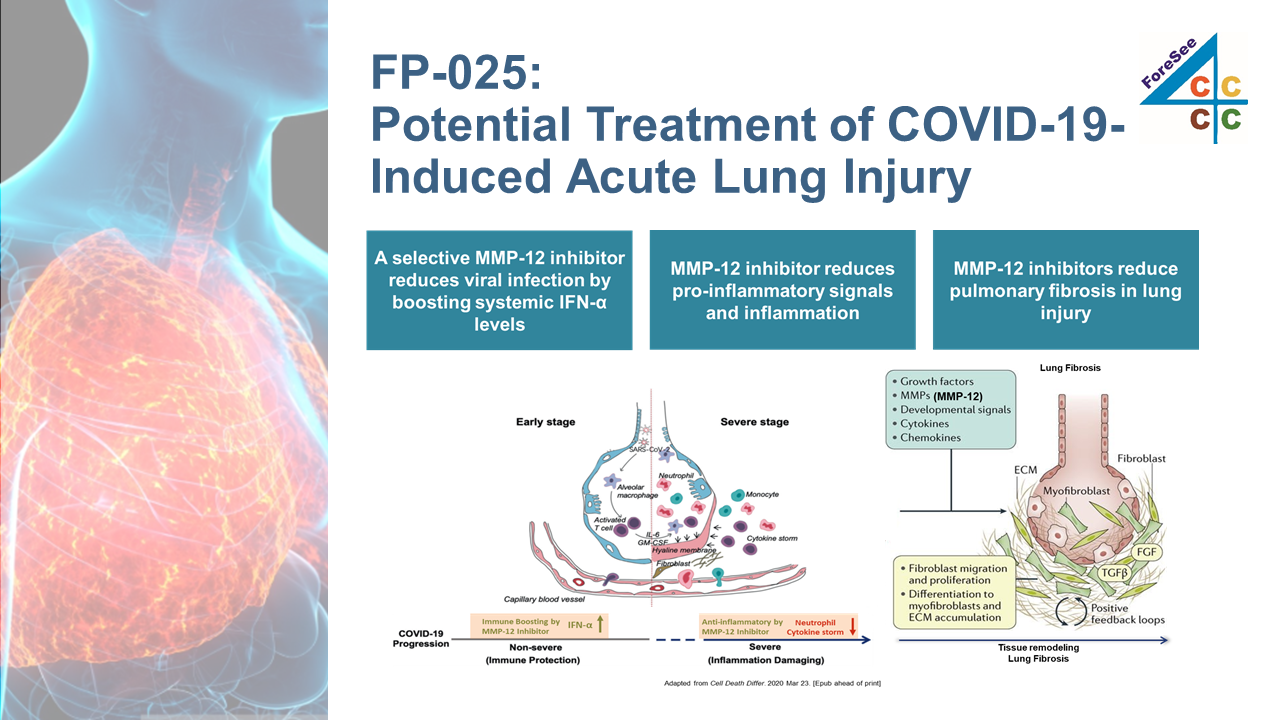
FP-025 is a highly selective small molecule inhibitors of Matrix Metalloproteinase-12/Macrophage elastase (MMP-12), was shown to have effects in preclinical inflammation and fibrosis models of the lung, making it a promising treatment for inflammatory airway diseases. FP-025 is currently in a P1b/2a proof-of-concept (PoC) study in allergic asthmatic patients challenged with an allergen (house dust mite).
Severe COVID-19 caused inflammation of the lungs may cause pulmonary fibrosis. Foresee Pharma plans to enter phase II clinical study being developed for the treatment of COVID-19 triggered ARDS and pulmonary fibrosis, after IND pre-meeting with FDA in Q4 2020.
FP-025 will be the first-in-class medicine of MMP-12 inhibitor, for the treatment of ARDS once obtained the NDA approval.
Epidemic Prevention Advantage:
Innovation, highly selective MMP-12 inhibitor, oral non-steroidal anti-inflammatory drugs, Inhibition of lung fibrosis, Potential Treatment of COVID-19-Induced Acute Respiratory Distress Syndrome.
Collaboration Options:Product/technical license, investment collaboration