Production of High Specificity Monoclonal Antibodies and Detection Kits for COVID-19 Infections
2020-11-10
Production of High Specificity Monoclonal Antibodies and Detection Kits for COVID-19 Infections
National Defense Medical Center
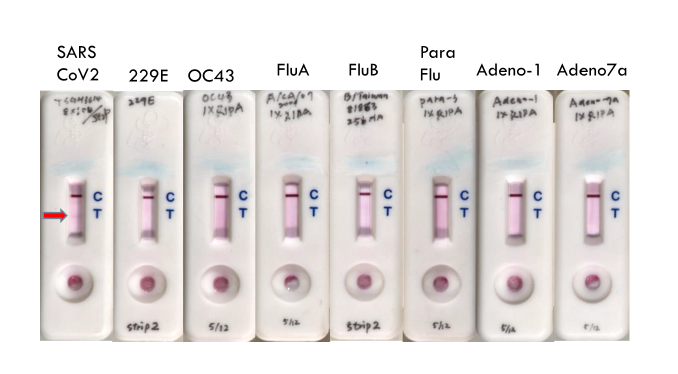
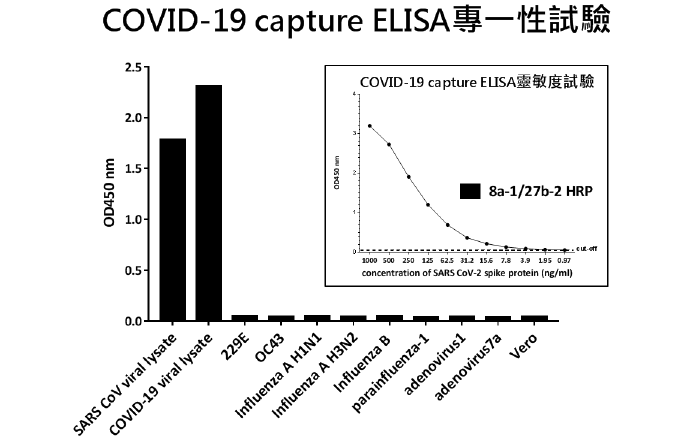
Our laboratory has developed two strains of hybridoma against SARS-CoV-2 surface spike proteins, named 8a-1 and 27b-2, which produce highly specific and stable monoclonal antibodies. Both antibodies can specifically identify the corona-virus S-2 protein sequence, and will not cross-react with N and E proteins. After testing, it has good protein stability. Using the pairing of the two monoclonal antibodies, we established successfully immunological detection kits for a lateral flow rapid test to detect SARS-CoV-2 virus in human specimens. In spiked sample, as low as 8 x 10,000 PFU virus of single drop would be accurately quantified. A rapid screening kit was developed that can quickly test viruses within 15 minutes. For potential infected persons, effective screening of large of samples will be performed. At present, a number of biotech manufacturers have strive for co-operation and invested in the production of fast screening kits to enter the global market.
Epidemic Prevention Advantage:
1. Two SARS-CoV-2 Spike protein hybridoma cells, stable secretion of
monoclonal antibodies.
2. Provide rapid screening test kit for COVID-19 test, and get the
result in 15 minute.
3. A large number of specimens can be screened to achieve the epidemic
prevention requirements.
Collaboration Options:Technology transfer and authorization