Hualien Tzu Chi Hospital
Cell Products Using Autologous Mesenchymal Stem Cells Bringing Hopes to Patients with Spinal Cord Injury

The team at the Gene and Stem Cells Manufacturing Center, Hualien Tzu Chi Hospital (Photo courtesy: Hualien Tzu Chi)
Stem cells repair soft tissues and organs, carrying much weight in the development of regenerative medicine. Medical centres from across Taiwan, on top of the advanced therapeutics they offer, have been delving into research and the use of stem cells for treatment.
Amongst them, Hualien Tzu Chi (“Tzu Chi”) Hospital has been spearheading stem cell treatment from treating patients to manufacturing cell-based products. In 2020, the very first treatment leveraging autologous mesenchymal stem cells (MSCs) on a patient whose spinal cord was damaged was proved to be a success under the care of Tzu Chi and its biotech partner, Taiwan Advance Bio-Pharmaceutical Inc. (TABP).
Two years later, the second case arrived, yet Tzu Chi went to great lengths to make MSCs production in-house this time with its proprietary “smart manufacturing” process, having built the Gene and Stem Cells Manufacturing Center to for the purpose. Co-located with Tzu Chi, the Center is where organisms derived from human bodies are prepared and handled to the highest safety and quality.
Sitting on the east coast of Taiwan, Hualien city is less regarded as convenient in terms of accessibility to healthcare providers as well as transportation. Tzu Chi is thus committed to turning the tables on health inequity with first-class patient care, setting a demonstration site in which its research can one day be clinically put into practice so as to develop novel treatments.
MSCs: From Outsourcing to One-Stop Solution
The second case involved a male patient, Mr. H, who was enthusiastic about motorcycles before he was struck by a severe accident on a road trip in 2022. His thoracic spine was then fractured, and he lost mobility and sensory functions, nor could he control his bowel and bladder. It was inevitable to get around with a wheelchair, as he had been told, although he had never given up seeking options for recovery.
When Mr. H learned that a patient was treated with MSCs by Tzu Chi and the outcome was positive, he came to the hospital a year later.
Tzu Chi operates a GTP lab within its Gene and Stem Cells Manufacturing Center, where stem cells are cultured and multiplied. To proceed with stem cell treatment, one must satisfy certain conditions. Mr. H had been thoroughly MRI-checked to identify the exact spot and level of his spinal cord injury, which attested to his eligibility for the treatment. His bone marrow would then be collected, cultured and administered through a medical procedure onto his lesion.
Dr. Sheng-Tzung Tsai, the lead neurosurgeon at Tzu Chi, explained Mr. H accepted surgery that aspirated 10c.c. to 20c.c stem cells from the remaining functional part of his spine. Those stem cells would then be isolated, pacificated and proliferated in about two weeks, during which cell counts should have reached 5 million for the making of a cell product. Once a cell product is filled and finished, it can be administered six times onto the upper and lower part of the spine that had already been paralysed.
Judging by the patient’s outcome and the success rates, the treatment at Tzu Chi outperformed what was traditionally applied to patients like Mr. H, namely rehabilitation therapy.
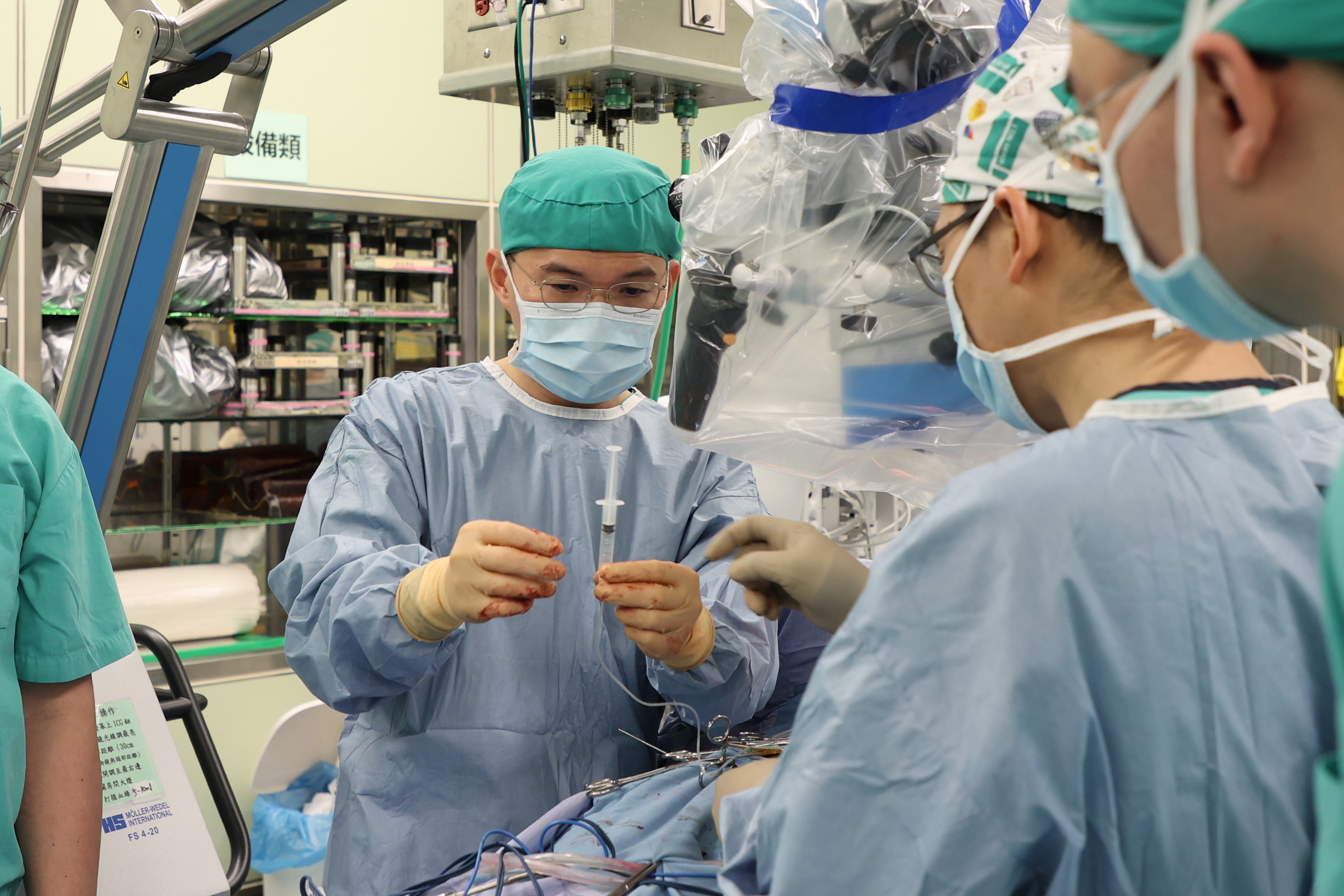
Finished cell products to be administered six times onto the patient’s spinal cord (Photo curtesy: Hualien Tzu Chi)
Well-Coordinated and Rigid Process of Stem Cell Treatment
Hualien Tzu Chi has been in the field of stem-cell-based study and treatment, inaugurating its GTP lab back in 2003. It was the first hospital in Taiwan equipped with a TFDA-certified facility for carrying out stem cell bioprocessing, manufacturing, and storage. The hospital is also the only one in the country that employs the “Cell Processing Isolator (CPi)”, which originated from Japan, to prepare mesenchymal stem cells.
Dr. Li-Yi Sun, the Director who heads the Gene and Stem Cells Manufacturing Center, stated that the coordination between the hospital, the patient and the lab is the key to success, and that, in a sense, outweighs the laboratory workflow itself.
Dr. Sun also explained how Tzu Chi’s stem cell treatment is prepared and delivered. It all begins two weeks before ready-for-use cell products are given to patients. An infusion procedure performed by Dr. Tsai, the neurosurgeon, is typically scheduled on Thursday morning. Bone marrow cells collection will thus be carried out by an oncologist two weeks earlier on Monday, during which the cells will be cultured, and the team at Tzu Chi will keep Dr. Tsai and a case manager informed of the status of cell culturing. In the meantime, the patient’s health will be closely monitored to assure he/she is clinically qualified for the infusion.
On the day before the procedure, the lab prepares for the release of the final product through several steps: tracking records on culturing and facilities, removing cells from the petri dish to prevent them from sticking together, performing activity and safety tests, processing the final product fill, and placing it in a temperature-controlled container. Since the above-mentioned steps require at least four hours, the release must start at least six hours before the surgery. All relevant information is double-confirmed with a case manager in early in the morning on the day of the surgery.

The Cell Processing Isolator (CPi) for the use of MSCs preparation (Photo curtesy: Hualien Tzu Chi)
The Cell Processing Isolator (CPi) Tzu Chi purchased, originated from Shibuya Corporation, Japan, is priced at TWD$30 million per unit, which is often sold to pharmaceutical companies for making sterile injections. It is a rare case for a hospital to invest that much money in such sophisticated equipment for early-stage developments, but Tzu Chi has a good rationale behind its investment. The hospital, situated in the east coast of Taiwan where no biotech clusters nearby can offer assistance, sees the necessity of having its own laboratory equipment in place not only to expand its R&D capacity but also to prioritise quality patient care.
The investment does not make the case for hardware only. The Gene and Stem Cells Manufacturing Center has employed a clinical laboratory scientist and a quality assurance engineer to create quality inspection tests and assist in deploying a laboratory information management system. Patients’ cells are confined in an isolator that is exclusive to one patient at a time, ensuring safety and quality whilst preventing contamination.
Integration of Traditional Chinese-Western Medicine and Mental Support Improving Patient Outcomes
Patients with spinal cord injuries treated with autologous mesenchymal stem cells only need to receive a single infusion of cell products, which costs about TWD$800,000 or so, with three follow-up visits scheduled in the first three months, and another one in the sixth month since the treatment to evaluate the patient’s outcomes.
Mr. H underwent surgery in March 2023, during which he was also treated by Dr. Yen-Lun Kung, a Chinese medicine practitioner, with acupuncture and electrotherapy. He also followed the instructions of his physiotherapist for rehabilitation. In July 2023, he began to feel sensations 10 centimetres below his chest, could feel the warmth and the urge to urinate from his lower back down, and was able to attempt to defecate. That was a significant improvement from his pre-surgery performance. He shared his story on social media, and people started sending him warm words of encouragement.
Dr. Sun, who heads the Gene and Stem Cells Manufacturing Center, commented that the volunteers and the rehabilitation team at Tzu Chi provide the support a patient needs, complementing stem cell treatment to maximise synergy altogether. They go the extra mile to help and encourage patients who might have given up on rehabilitation after the treatment, he added.
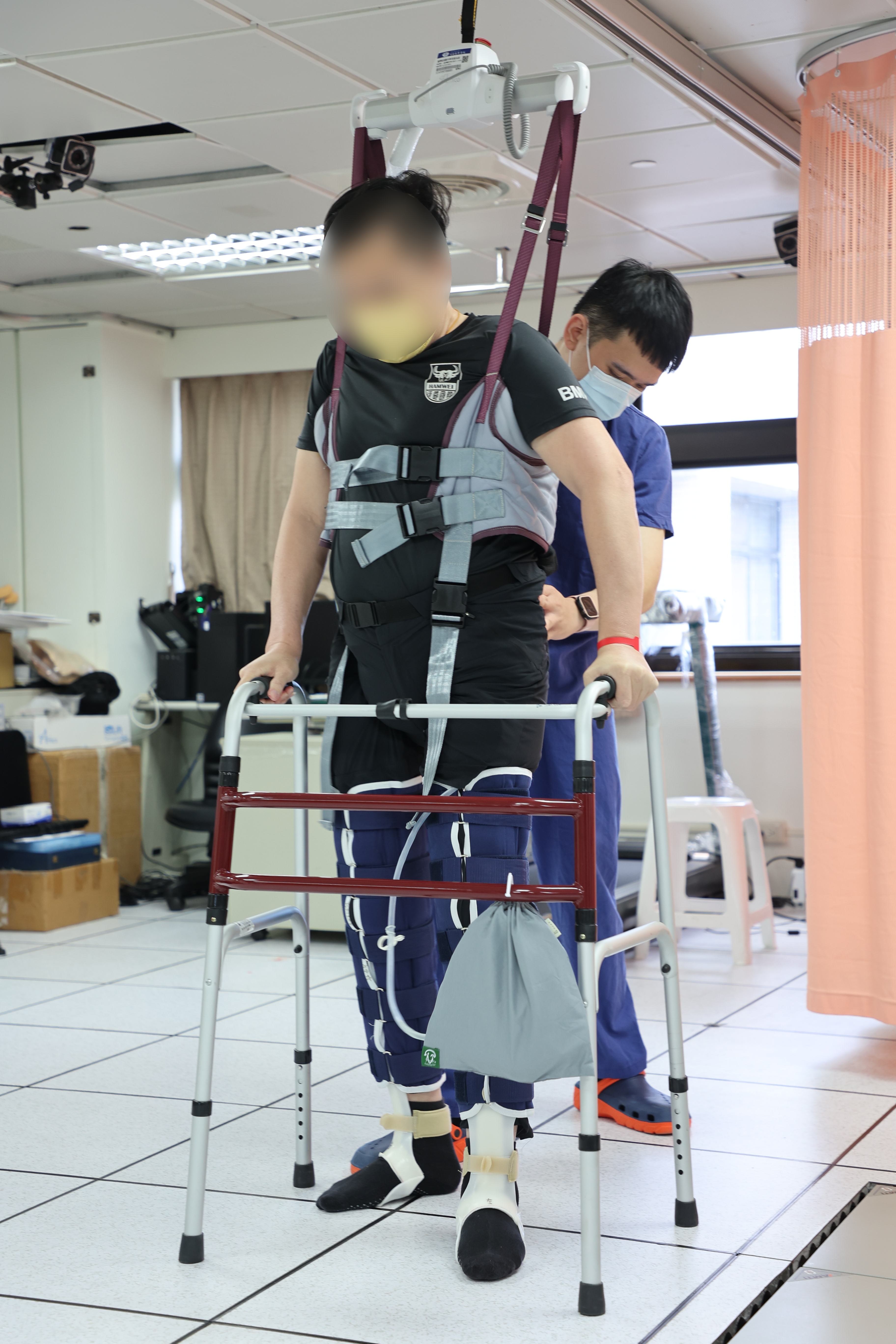
The patient who was rehabilitating with the help of a physiotherapist (Photo: Hualien Tzu Chi)
‘Smart’ Manufacturing, Paperless Management
Cell products for use in stem cell treatment are tailored and individualised, usually accompanied by a substantial pile of paper-based medical records that, by law, must be kept for 10 to 30 years. To address that, Tzu Chi deployed a ‘smart’ manufacturing system developed by Shibuya Corporation in Japan and worked to meet PIC/S GMP standards. Initially, a paperless record-keeping system was introduced, followed by automated duty rostering and equipment scheduling systems. These systems tracked and logged details such as donors and quantity of cells, where and how cells were processed, how long an equipment was switched on, and how often consumables were replaced. This thorough and detail-oriented approach reduces error-prone factors, improving safety and quality of the treatment to the largest extent.
Should the number of patients increase and more demands for cell manufacturing arise from Tzu Chi’s branch hospitals, it will be time to introduce a robotic-assisted, automated manufacturing system to fulfil “smart manufacturing” commitment that Tzu Chi has for benefiting patient care.
Dr. Shinn-Zong (John) Lin, the Superintendent of Hualien Tzu Chi, expressed gratitude for what they have achieved in stem cell treatment, which is now gaining popularity amongst patients from elsewhere in Taiwan. “Our aim in the beginning was to address health inequity on the east side of Taiwan. Hualien Tzu Chi is now a place where dreams come true for patients”, said Dr. Lin.




