
Covid-19: Three strategies for alleviating healthcare workloads and public panic
2021-05-24
The recent surge in new cases reported in Taiwan has increased challenges for contact tracing, the ER unit has now nearly crippled by a long line of the public panicking and wishing to get a diagnostic test of whether they are at risk of infection. Workloads have been piling up to the extent which healthcare providers, public health authorities and laboratories can barely handle on a daily shift. Simultaneously, demands for diagnostic tests and personal protective equipment have soared with the ensuing community transmission.
The Institute for Biotechnology and Medicine Industry (IBMI) hereby proposes the following strategies in hope of addressing the issues flagged by healthcare workers and easing panic.
I. Roll out with accurate, rapid and high-throughput RT-PCR tests
The imperative would be for Taiwan to curb SARS-CoV-2 transmission and increase provision of mobile, massive RT-PCR tests. Tests developed with antigen and antibody have been proved with test results suggesting false negative or false positive, which has to be justified eventually with PCR. Now with AI, the virus can even be detected in five minutes from a test taker’s saliva.
There have been technologies and suppliers available in country to satisfy varying needs from test kits, examinations through to test stations, the nation should call on a team in place offering mobile, massive RT-PCR tests- with the Ct value generated and shown in a test result- ideally within an hour. In epicentres and national borders, the roll-out of RT-PCR test will ring fence further risk of COVID transmission from and to Taiwan.
II. Centrally-coordinated resources needed by the health system
The private sector can help, though, it relies on a central command force to communicate where and what the demands are and will be. IBMI had leveraged its connections to have consolidated a response with health supplies that hospitals seek the most, which includes:
1) Detection of COVID: test stations and equipment, rapid-test kits and PCR reagents.
2) Personal protective equipment: N95 face masks, goggles, water-proof isolation gowns.
3) Telemedicine: biosignal monitoring devices for mild cases staying home.
4) Video conferencing: relevant devices and systems for diagnosis, meetings, and medical counseling.
III. Publicly available updates on epidemic management products
The outbreak of Covid-19 since last year has facilitated development of a broad range of products and solutions, resulting in an overall increase in R&D investment and wider medical coverage for those successful ones brought to market. Despite having a list by TFDA of medical devices eligible for EUA, the status of COVID-related drugs and vaccines being developed or approved appears to be inconsistent. One publicly available, single source of information on epidemic management technologies, therapeutics and other applications can be provided for the public to access and get a holistic picture of Taiwan’s strength areas in tackling the public health crisis.
Different Types of COVID Tests (source)
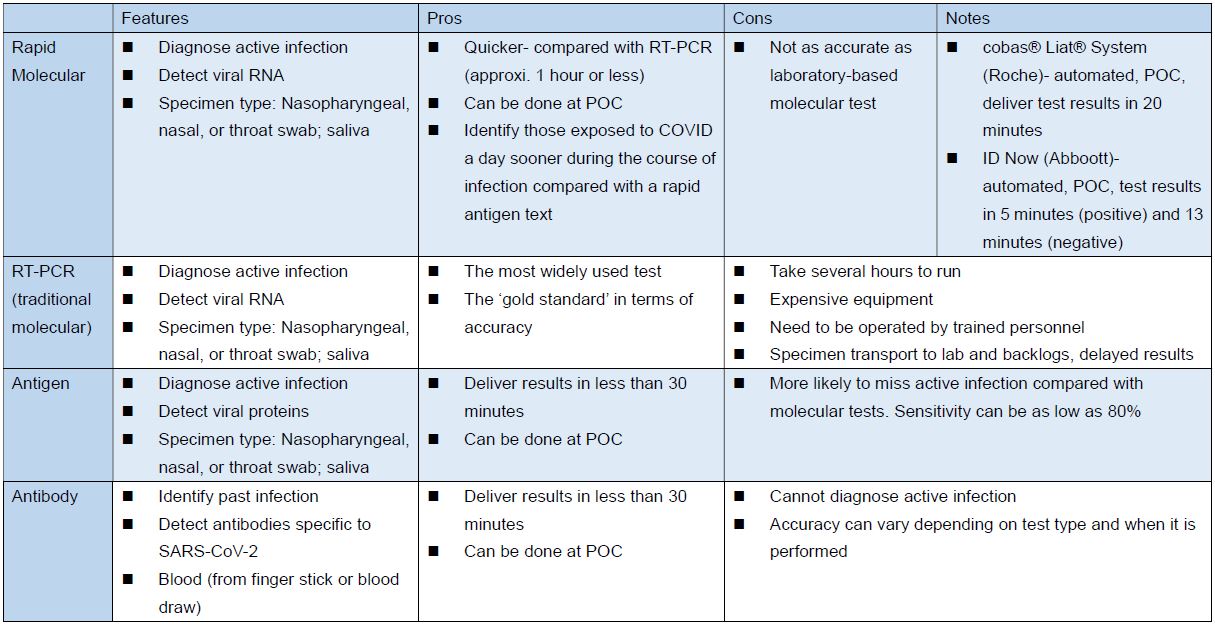
Source: FDA, the Pew Charitable Trusts: COVID-19 Tests Highlight Need for Strengthened FDA Oversight and Diagnostics Legislation; the table above compiled and edited by IBMI.