Non-steroidal anti-psoriatic botanical drug PTB323X
2020-11-06
Psoriasis affects approximately 2-3% of the world‘s population and is an autoimmune disease and a chronic inflammatory skin disease as a serious global problem. As first-line treatment for psoriasis, corticosteroids often possess serious long-term side effects. Therefore, the most urgent need for psoriasis therapy is developing drugs for long-term use with high efficacy and little side effects. PTB323X, a new botanical drug for the treatment of psoriasis, is developed by the Botanical Drug Technology Division of the Industrial Research Institute. In addition to effectively improving the symptoms of psoriasiform dermatitis in animals, the efficacy of PTB323X is superior to vitamin D derivative and has no skin-related side effect during the experimental period.
The PTB323X has high maturity, and has the following characteristics
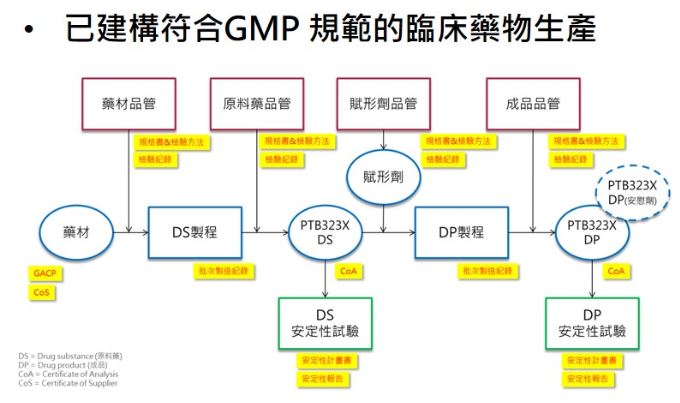
- The cultivation process has been constructed with GACP.
- The CMC process has been followed by GMP.
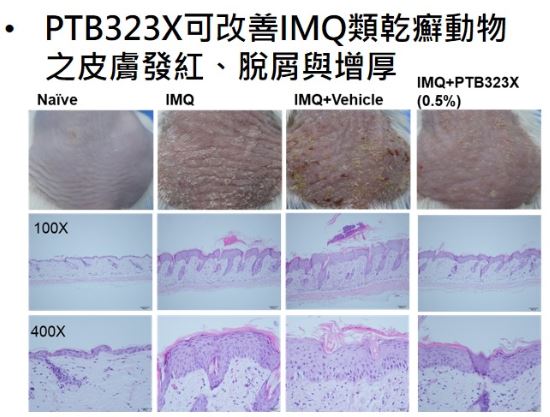
- PTB323X ointment has the effect of improving skin redness, scaling and thickening of the skin of animal psoriasis, and the overall effect is better than Daivonex® (Vit D derivative)
- GLP toxicology and pharmacokinetics have been completed and explored the mechanism of action
- TFDA conditional approved Phase I/II clinical trial in 2020
- The development technology of PTB323X has been applied for 10 patents in 3 cases in Taiwan, US, China, EU and Japan. Also, 4 international conferences and papers have been published
InnoZone