Leadgene Biomedical Pioneers Globally with Patented Chronic Kidney Disease Detection Technology, Leading in Antibody Research
2023-11-06
Source: Leadgene Biomedical, Inc. ( Healthcare+ Expo- booth no.: M319a)
Pioneering Antibody Development: The Success Story of Leadgene Biomedical
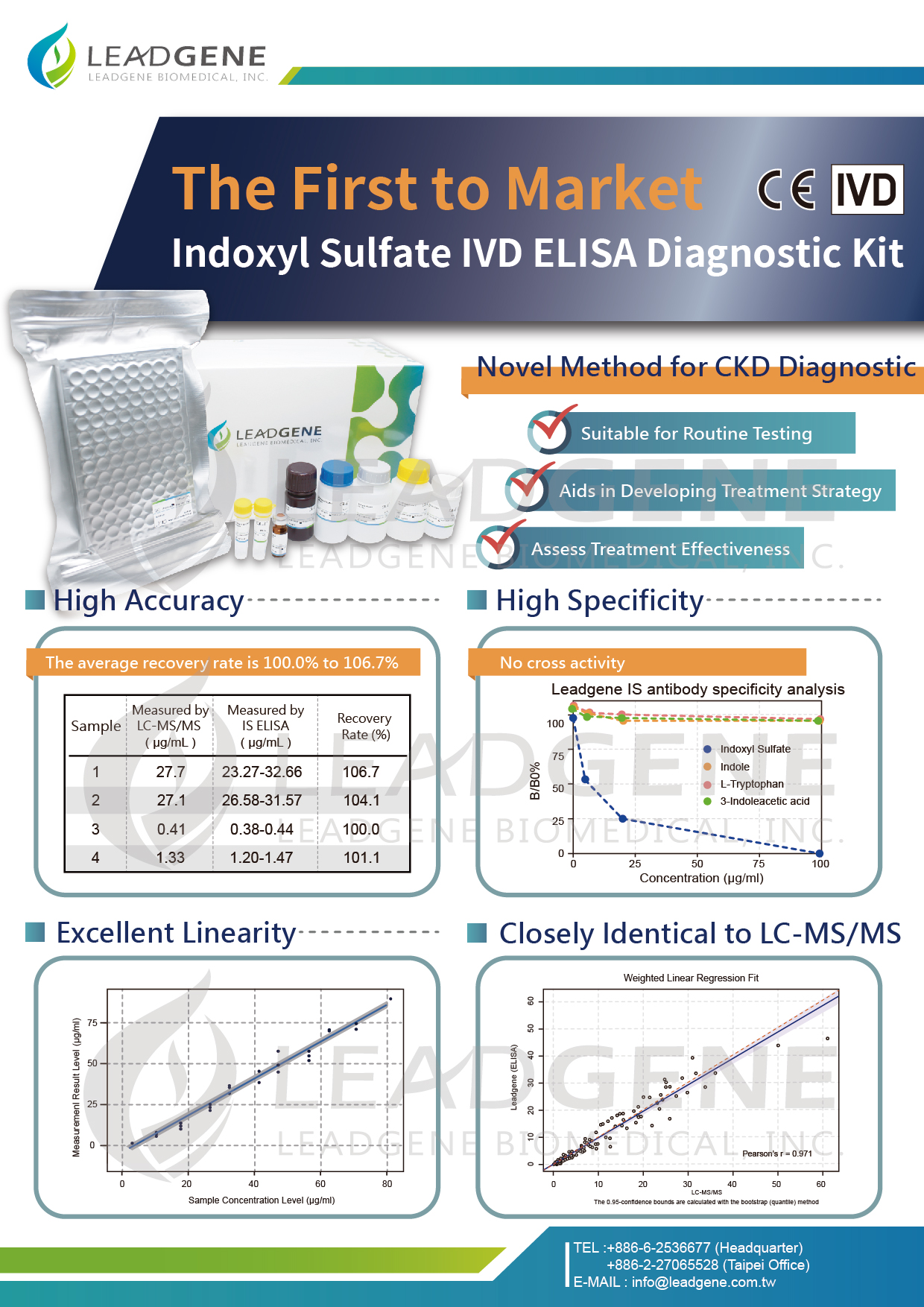
Leadgene Biomedical, Inc., at the forefront of recombinant protein and antibody manufacturing, prides itself on a proficient R&D team and a stringent quality control system validated by ISO13485 and QMS certifications. The company offers extensive reagent platform development services, encompassing the development of lateral flow immunoassay (rapid test) platforms and ELISA platforms. Leadgene has autonomously developed and manufactured an Indoxyl sulfate (IS) ELISA Kit, marking it as the first protein-bound uremic toxin detection kit available on the market. This kit facilitates the swift, convenient, and high-throughput detection of one of the uremic toxins, indoxyl sulfate, in patients, offering clinicians and patients enhanced renal function indicator information for early diagnosis and treatment. It finds extensive application in clinical research, kidney disease screening, disease progression, and treatment assessment. Having passed EU CE certification and being submitted for medical device inspection and registration in Taiwan, the detection kit is also seeking approvals in various countries across the Asia-Pacific region, aiming to benefit patients with kidney diseases significantly and those undergoing dialysis, as well as being utilized in routine physical examinations and preventive medicine.
A Global First: Patented Antibody that Breaks Technological Barriers
With a persistently high number of individuals undergoing kidney dialysis, chronic kidney disease has remained atop Taiwan's list of most financially draining chronic diseases for numerous years. Protein-bound uremic toxins, such as indoxyl sulfate or p-cresol, pose a latent threat by accumulating in the blood and potentially causing visceral damage. Numerous domestic and international studies have identified a correlation between increased concentrations of indoxyl sulfate and kidney damage, which can lead to cardiovascular calcification, myocardial cell fibrosis, and other conditions. These molecules, which readily bind with proteins in the blood circulation, are challenging to detect and require high-specification instruments like LC-MS/MS. The Indoxyl Sulfate ELISA Kit, developed by Leadgene Biomedical, stands as the world’s first and most efficient reagent for detecting a crucial indicator of chronic kidney disease. It has shattered the bottleneck of small molecule antibody development, pioneering the antibody of protein-bound small molecule uremic toxin and securing patents in several countries. This kit offers a faster, more convenient, and high-throughput alternative to the expensive and time-consuming traditional clinical detection method LC-MS/MS, providing clinicians and patients with additional indicators of renal dysfunction and enabling early disease treatment.
Rapid and Efficient Detection: Aiding Patients with Chronic Kidney Disease
Leadgene Biomedical Indoxyl Sulfate ELISA Kit offers a viable alternative to costly and inconvenient high-specification instruments, enabling convenient and rapid large-scale detection of indoxyl sulfate in patients, and facilitating early kidney disease prevention. For patients with chronic kidney disease, this kit can be utilized to monitor disease progression and evaluate renal function improvement pre and post-medication. It can be widely employed in clinical research, kidney disease screening, disease progression, and treatment assessment. Leadgene Biomedical, having successfully developed a detection kit targeting indoxyl sulfate, aspires to provide kidney disease patients with a quicker, simpler, more precise, and affordable detection method. By connecting industry and experts through products and core technologies, Leadgene Biomedical propels Taiwan into a leadership position in the development industry of detection reagents, antibodies, and recombinant proteins, showcasing Taiwan's prowess in the biomedical field on the international stage.